WelcomeWelcome to CMAC News. If you have any news items for the next edition please contact helen.feilden@strath.ac.uk. |
||||
|
ISPE Facility of the Year Award CMAC's National Facility within TIC was awarded an Honourable Mention at the ISPE Facility of the Year Award (FOYA) 2016 banquet held on Tuesday 7th June outside Washington DC. This is the first time an academic institute has been recognised by these awards. The banquet was held in conjunction with ISPE/FDA/PQRI Quality Manufacturing Conference. CMAC was represented by Dr Andrea Johnston, Dr Stewart Mitchell, Vishal Raval, Dr Naomi Briggs, Scott McPhee and John Mulgrew. FOYA is the premier global awards programme recognising innovation and creativity in facilities serving the regulated healthcare industry. The FOYA judges felt the project was well worthy of an honourable mention due to the exemplary collaboration between industry, academia and government which represents the future of pharmaceutical manufacturing and supply chain R&D framework. In addition, the students working with such technology and in such a collaborative environment will be the pipeline for the pharmaceutical professionals of the future. |
 |
|||
|
|
||||
Andrew Witty VisitSir Andrew Witty, chief executive officer (CEO) of GlaxoSmithKline visited the CMAC National Facility in February 2016. There were presentations and discussions on the research, skills, facilities and key industry translation activities followed by a tour of the facilities. He was accompanied by the Principal of University of Strathclyde Professor Sir Jim McDonald, Scottish Enterprise and senior representatives from GSK. Sir Andrew and his team were very complimentary and were impressed by the range of activities and people they met. He commented that CMAC was an exemplar of critical mass research activities. |
 |
|||
|
|
||||
Siemens CEO Visits TICJuergen Maier, CEO of Siemens UK, visited the Technology and Innovation Centre (TIC) as part of a University of Strathclyde wide event. During his visit in March 2016 he toured the EPSRC Centre for Innovative Manufacturing in Continuous Manufacturing and Crystallisation (CMAC). During Juergen's visit he reaffirmed Siemens commitment to supporting CMAC to the Principal Prof Sir Jim McDonald. In the picture from left to right: Phil Shering - AstraZeneca Associate Engineering Director; Juergen Maier; Prof Sir Jim McDonald, Principal; Craig Johnston, CMAC Industrial Director; and Prof Alastair Florence, CMAC Director. Siemens plc are now members of CMAC alongside established Tier 1 and Tier 2 members, which also include Siemens approved Solution Partner - Booth Welsh. Juergen presented to a large audience in the Main Auditorium, and presented a fascinating talk in industrial history, the current status and a future vision for industry in the UK and Scotland entitled "Can Scotland seed a new Industrial Revolution?". |
 |
|||
|
|
||||
Wolfson LabPaul Ramsbottom, CEO of The Wolfson Foundation, visited the Technology and Innovation Centre alongside Mr Glen Watson (MBA). Paul’s visit included a visit to the TOF-SIMS in the newly named "Wolfson Pharmaceutical Surfaces Laboratory", whereby Prof Alastair Florence and Dr Dimitrios Lamprou gave him a brief explanation of its features, the benefits towards Centre research and thanked the foundation for their generous donation towards excelerating CMAC’s capabilities. |
 |
|||
|
|
||||
ADDoPTThe ADDoPT (Advanced Digital Design of Pharmaceutical Therapeutics) project addresses a key challenge for the pharmaceutical industry: getting new innovative medicines to market in the quickest and most cost-effective way possible to ensure access for patients. CMAC are specialist knowledge-based partners in this project with CMAC’s Prof Alastair Florence being a Co-Investigator and Dr Blair Johnston being technical lead in this project. For more information please click here. |
 |
|||
|
|
|
|||
EU Grant SuccessIn March 2016, CMAC’s Prof Joop ter Horst obtained funding for a 4 year postdoc within the Fet-Open 2014/2015 call as part of the AMECRYS consortium (€335,500). The Horizon2020 Fet-Open consortium funding opportunity is a very competitive funding for fundamental research. This year only 11 proposals were funded out of 669 proposals. AMECRYS aims at revolutionising the manufacture of biopharmaceuticals with innovative membrane crystallisation technology. More here The field of chiral resolution & deracemization will become increasingly important for the pharmaceutical industry and CMAC. This is exemplified by the funding Prof Joop ter Horst obtained to set up a Marie Sklodowska-Curie Innovative Training Network on Continuous Resolution and Deracemization of Chiral Compounds by Crystallisation (CORE). This European network brings together 15 PhD students, 8 academic and 7 industrial partners from 6 European countries to jointly construct an Industrial Toolbox on Continuous Resolution that provides next generation tools, approaches and methods to industry for the development of continuous resolution processes. |
|
|||
|
|
||||
Chief Scientific Officer Visits CMACProfessor Sir Mark Walport (Government’s Chief Scientific Advisor) and Dr Neil Waby (Climate Change & Energy Policy Adviser) visited CMAC’s X-ray Suite earlier this year where they met key CMAC people and were introduced to our world leading suite of instruments on structural characterisation and analysis and the role it plays in manufacturing and pharmaceutical development. |
||||
|
|
||||
CMAC in UK Manufacturing ReviewCMAC has been featured in the UK Manufacturing Review 2015-2016. The publication is a 250+page high quality book, a “super-magazine”, written by expert journalists across industry sectors, that summarises the status of the manufacturing, engineering and technology sectors in 2015. CMAC was featured on p220 of the Review as well as being mentioned in the "Road to 2016" infographic on p18 and the section on Pharmaceuticals on p43 of the book.
A Manufacturing Future for ScotlandCMAC is featured in “A Manufacturing Future for Scotland”. There is a case study on Syngenta and CMAC on p37 of the Review. The review can be found here |
 |
|||
|
|
||||
Engage With Strathclyde |
||||
CMAC Tier 2 Engagement EventOn 4th May we held an event at Engage With Strathclyde - Collaboration with the Centre for Continuous Manufacturing and Crystallisation (CMAC). This event was designed to bring together CMAC Tier 2 companies and our academics and researchers to explore how we can collaborate moving forward. There were 26 flash presentations hosted within half a day to give an excellent overview of the projects, technology and collaborations between the Tier 2 Technology companies and CMAC researchers. We also had a pre event networking dinner around the Images of Research where discussions took place, and an afternoon event where PwC hosted and presented an excellent showcase. Thanks to all the companies, researchers, academics and Engage with Strathclyde who made this event a great success. Contact: stewart.mitchell@strath.ac.uk |
 |
|||
Engage with Science: Boost BusinessCMAC was also represented at the Engage with Science: Boost Business science faculty event where Centre Manager Dr Andrea Johnston gave a talk, “Centre for Innovative Manufacturing in Continuous Manufacturing and Crystallisation (CMAC)”. |
||||
Hyperspectral Imaging: Monitoring Continuous Production of Pharmaceuticals and Fine Chemicals (ICT-CMAC Project)The CMAC National Facility was showcased as part of the ICT CMAC demonstrations that took place as part of Engage With Strathclyde. Each one-hour demonstration included a short introduction to the project, a showcase of the hyperspectral imaging method, followed by a tour of the world-leading facilities. |
||||
|
|
||||
DEM: New Tier 2CMAC has chosen EDEM® simulation software to support research and training in advanced pharmaceutical manufacturing. DEM Solutions, the Discrete Element Method (DEM) specialist and market leader, announced it has joined CMAC as a tier 2 industry partner and will provide its EDEM software to integrate into the Centre’s research program. More information can be found here DEM is an Edinburgh based company who have developed and market Discrete Element Method software used for bulk material flow simulation, this is used for modelling and virtual testing of equipment that handles or processes bulk materials in manufacturing and process industries. Dr Blair Johnston is the key researcher who will be working with this new application and DEM are kindly supporting CMAC with the provision of their software and company support for its application. |
 |
|||
|
|
||||
National Facility Open for BusinessCMAC has established a National Facility to work extensively, not only with academic institutions, but also with SME and Multi-National companies to provide a fee for service for collaborative projects, ranging from process development to analytical services. The National Facility team will be exhibiting at CPhI Worldwide conference in Barcelona, Spain (4-6 October 2016) to provide information and capabilities for attendees. In addition, Craig Johnston, CMAC Industrial Director, will be presenting during the pre-connect congress in a section entitled "Driving innovation and competitiveness in formulation, sourcing and manufacturing strategies". Enquiries to the CMAC National Facility team can be made using the following link. |
 |
|||
|
|
||||
Outreach |
||||
AcademicProfessor Sally Price, (UCL and CPOSS www.cposs.org.uk) visited Strathclyde in early 2016 and met with some researchers as well as delivering an RSC lecture entitled "Is the crystallisation of pharmaceutical molecules controlled by thermodynamics or kinetics?". Dr Harris Makatsoris of Cranfield University visited Dr Andrea Johnston and CMAC colleagues in TIC on 27th April 2016 and as part of his visit he delivered a seminar: “Robot chemists, artificial creativity and self-evolving chemical systems: the evolution of science or science fiction?” Dr Steven Ferguson has recently taken up a position as a Lecturer in the School of Chemical and Bioprocess Engineering, at University College Dublin and visited CMAC’s Prof Jan Sefcik on 12th May 2016. As part of the visit Steven gave a seminar entitled “Continuous Crystallization of Active Pharmaceutical Ingredients”. |
|
|||
I2APM |
||||
|
The International Institute for Advanced Pharmaceutical Manufacturing brings together world-leading academic expertise to deliver new end-to-end continuous manufacturing capabilities that will transform the global supply chain for medicines. Founded by the Centre for Innovative Manufacturing in Continuous Manufacturing and Crystallisation, CMAC (UK), the Centre for Structured Organic Particulate Systems C-SOPS (US) and the Research Center Pharmaceutical Engineering, RCPE (Austria), the joint programme leverages existing extensive investments in the UK, US and EU by creating a vibrant international manufacturing research community that will accelerate progress through excellence in research. Furthermore, by engaging with regulators the research will be targeted to maximise impact for end users. Plans for SymposiumThis year CMAC will host the first I2APM international symposium, which will take place on 30th November 2016. This will be followed by a day of training, comprising sessions from CMAC, C-SOPS and RCPE. The symposium will be an internal I2APM event whereby the staff and students from all three centres will come together to learn about research being carried out across all institutions. This will be a great opportunity for our centres to really get to know each other, and will help to establish further areas of complementary collaboration and researcher exchanges. Website LaunchThe I2APM website is now live, and can be accessed at www.I2APM.org. The website is currently a work-in-progress, and more information about I2APM and its activities will follow in the next few months. |
 |
|||
Visit by Director of Scientific Operations at RCPECMAC hosted a visit from Massimo Bresciani, Director of Scientific Operations at RCPE, Austria. |
|
|||
Visit by Professor San Kiang, C-SOPSIn May 2016, CMAC hosted Professor San Kiang from C-SOPS. Prof. Kiang met with staff to discuss I2APM collaborative projects and was also shown round the CMAC laboratories and equipment. Prof. Kiang also gave a highly informative seminar for CMAC staff and students entitled “The Role of Particle Engineering in Designing Pharmaceutical Particles and Pharmaceutical Composite Material (PCM)”. |
 |
|||
Continuous Manufacturing Technology Roadmapping WorkshopIn June 2016, Craig Johnston, Industry Director at CMAC and Dr Cameron Brown, Senior Instrument Scientist for Continuous Processing, attended the Continuous Manufacturing Technology Roadmapping Workshop, co-hosted by C-SOPS and USP and held in Washington. Craig and Cameron attended presentations from academic, industry and regulatory delegates, and Cameron also participated in a panel discussion on the following topic – “Identify Achievable Target for API CM, Assessment of Capabilities, Gap Analysis and How to Overcome Barriers”. |
||||
ECCPM Workshop on Process ControlIn July 2016, Dr Cameron Brown, Senior Instrument Scientist for Continuous Processing, and Dr Claire MacDonald, National Facility Business Development Manager, attended the ECCPM Workshop on Process Control, hosted by RCPE in Graz, Austria. In addition to the workshop, Cameron and Claire met with Centre Director Johannes Khinast, Director of Scientific Operations Massimo Bresciani and Ass. Prof Heidi Gruber-Woefler, and were given a tour of the facility to see the new RCPE pilot plant. |
||||
Purdue PlacementIn Spring 2016 John McGinty went on secondment to Purdue University for 2 months. He spent the time involved in a combination of experimental and modelling work to learn new approaches and techniques used in Prof Zoltan Nagy’s group. John is working on PAT based monitoring of the crystallisation of an organic polymorphic salt and the subsequent estimation of its crystallisation kinetics. In particular he utilised PAT to monitor the crystallisation of his model compound, ethylenediamine 3,5-dinitrobenzoate (EDNB), and will use the data obtained to estimate its crystallisation kinetics in collaboration with Dr Qinglin Su. |
 |
|||
Secondment to CMACAss. Prof Heidi Gruber-Woefler (RCPE) will be on secondment at CMAC from September-December 2016, and will be working on a collaborative project with CMAC reasearchers. |
||||
C-SOPS WorkshopCMAC DTC researchers Alice Turner and Sarahjane Wood (both Halbert group, Strathclyde), and Research Associate Akos Borsos (Reilly group, Loughborough) participated in a course with C-SOPS at Rutgers University in New Jersey (June 2016). They learned about PAT, modelling tools and material analysis and characteristics associated with secondary processing. |
||||
| |
||||
Esteem |
||||
Connecting Early Career ChemistsDr Thomas McGlone of CMAC was part of the organising committee for the recent Royal Society of Chemistry Early Career Symposium (ECS) 2016. Thomas has written an article about this experience which can be accessed here. |
||||
CMAC @ #PBPWorldMeetingThe 10TH WORLD MEETING on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology took place in Glasgow from 4-7th April 2016. CMAC was one of the organisations involved with CMAC Director Prof Alastair Florence being a member of the organising committee for the conference and also chairing an afternoon session on Tuesday. CMAC researchers attended the meeting and the photo shows CMAC DTC Students Elanor Brammer and Laura Martinez Marcos presenting their posters. |
 |
|||
Invited SpeakersCMAC Director Professor Alastair Florence presented a case study “Development of Continuous Crystallisation Processes for Consistent Crystal Products” at 7th Annual Global Drug Delivery & Formulation Summit & Expo in Berlin on 23-25th May 2016. More information on the meeting can be found here. CMAC Industry Director Craig Johnston gave the keynote lecture at the SFL and BSCC Basel Joint Event on Life Science Innovation in Europe event on 31st May 2016. The event was a unique opportunity to hear updates from key experts at the forefront of activities in Life Sciences. Dr Keddon Powell was an invited speaker at the Africa Pharma Congress in Cape Town, South Africa, June 20–22nd. The theme of this conference is "Developing New Formulations And Efficacious Products”. |
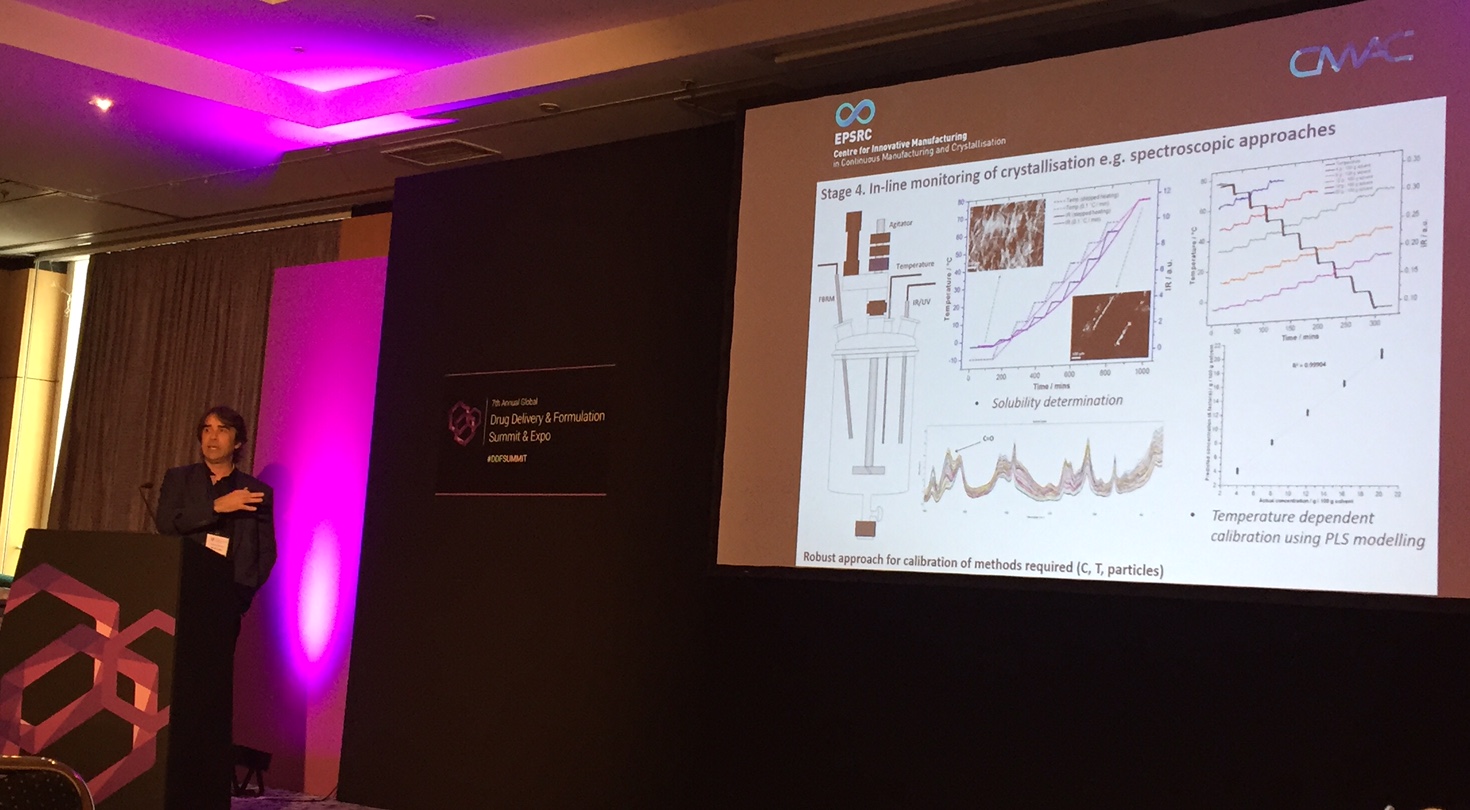 |
|||
PrizesCongratulations to CMAC DTC researcher Vaclav Svoboda who is the overall winner at this year’s University of Strathclyde Images of Research Competition. Vaclav who is in Professor Jan Sefcik’s group, had won the “Innovative Engineering” category for his image “Hidden Colours of Crystallisation” which was taken using the Leica DM6000 M microscope housed in the CMAC National Facility. Well done also to research associate Dr Rene Steendam, who is in CMAC Academic Prof Joop Ter Horst’s group, and was runner up in the “Size Matters” category with his Image “Manufacturing the Right Crystals”. CMAC DTC researcher Meifen Jiang’s photograph “Bee Crystals” has been awarded the 2nd prize in the 2016 EPS PGR Photography Competition at Heriot-Watt University. The photo is a microscopy image from Meifen’s work on crystallisation of paracetamol. CMAC DTC researcher Lauren Agnew (Wilson group, Bath) won a prize for best talk at the recent University of Bath Chemistry Postgraduate Symposium, for her presentation “Towards Multi-component Templated Continuous Crystallisation of Paracetamol Form II”. CMAC DTC researcher Dimitris Fysikopoulos (Rielly group, Loughborough) won the 2nd Poster Prize during the 4th Quality by Design Symposium that took place in Leicester on 15-16th March 2016. (The Poster Competition was sponsored by GlaxoSmithKline). The title of the poster was ''Population Balance Modelling of Continuous Crystallization Processes''. CMAC research associate Dr Keddon Powell has also won £1000 Santader Staff Mobility Grant at University of Strathclyde. The grant supported a 1-month visit to Purdue for knowledge exchange and development of expertise in continuous crystallisation for pharmaceutical drug development and will benefit both the UK and worldwide Pharma Industries. |
|
|||
AppointmentsCMAC academic Prof Chick Wilson has been appointed to the leadership team of the interdisciplinary Institute for Policy Research at the University of Bath, where he will lead on the interface between STEM research and public policy. Chick has had a longstanding interest in the interactions of science and engineering research with the policy context, and this role gives him the opportunity to assess both the ways in which such research can best influence policy, but also how STEM research can have best impact and influence within the policy and policy-maker context. Details of the appointment are given here (http://www.bath.ac.uk/ipr/news/news-0229.html), and more info about the IPR can be found here (http://www.bath.ac.uk/ipr/about/index.html). |
 |
|||
Participation in Workshops and EventsCMAC Centre Manager Dr Andrea Johnston attended Digital Technologies for Manufacturing Innovation: Embracing Industry 4.0. The event was organised by the Institute for Advanced Manufacturing (ifAM) at The University of Nottingham, in conjunction with EPSRC and Innovate UK. CMAC’s Prof Joop ter Horst and Dr René Steendam attended the Crystallize COST Action meeting in Berlin in late 2015. COST is a unique networking platform where European researchers can jointly develop their own ideas and new initiatives across all scientific disciplines through trans-European networking of nationally funded research activities. Prof Joop ter Horst is the vice chair of this action. The 2015 BCA Industrial Group Autumn meeting took place at AstraZeneca, Macclesfield. The day consisted of seven talks from various academic and industrial speakers, themed around the issue of “Cracking Challenging Crystals”. Anneke Klapwijk, Ruth Lunt and Alex Cousen, all DTC researchers in the Wilson Team at Bath, attended the event and found the day was extremely informative and enjoyable, outlining many novel techniques used for structural assessment, as well as demonstrating some industrial perceptions towards crystallisation. |
|
|||
|
|
||||
Researcher Visits and Placements |
||||
AstraZeneca PlacementFinal year DTC researcher Iyke Onyemelukwe (Rielly group, Loughborough) went on placement with AstraZeneca for 3 months in early 2016. He worked at the Macclesfield site working with Dr Anna Parsons and Dr Helen Wheatcroft investigating the continuous crystallisation of AstraZeneca compounds in the continuous stirred tank crystallizer (CSTR). |
 |
|||
AZ Remedies PlacementDTC researcher Sara Ottoboni (Price group, Strathclyde) went on placement for 2 months in early 2016 with AZ Macclesfield. As part of the REMEDIES programme a new lab scale continuous drug substance filtration and washing unit has been developed by Alconbury Weston Ltd (AWL). The overall aim of Sara’s project is to develop active pharmaceutical ingredient (API) filtration, washing and drying strategies that reduce processing time and cost and minimise negative impact on API physical properties to render the API optimal for secondary processing into drug products. |
 |
|||
Visit to AZDr Keddon Powell presented his work on the mathematical evaluation of a batch crystallisation process to an AstraZeneca advisory board in early 2016. A population balance model was developed using PSE’s gCRYSTAL 4.2 software in order to provide a better understanding and improve the performance of an AstraZeneca batch process. As part of the technology transfer, Keddon presented the model development framework and demonstrated the use of gCRYSTAL to AstraZeneca staff. The reviews of the work were very positive and AstraZeneca now has an appreciation of the benefits of applying process modelling to improve their crystallisation processes. |
 |
|||
Bayer PlacementFinal year DTC researcher Anneke Klapwijk (Wilson group, Bath) completed a two month placement at Bayer in Germany in early 2016. She worked in Leverkusen for four weeks in Formulation and Crystallisation with Dr Wolfgang Beckmann, Dr Michal Sowa and Dr Guillaume Levilain investigating the effect of additives on crystal habit. This was followed by four weeks in Wuppertal working with Dr Britta Olenik and her team on polymorph and salt screening using multiple solid form characterisation techniques. Anneke enjoyed working in an industrial environment, giving her the opportunity to apply her skills and learn new techniques. She also found the chance to live in a different country a great experience and would recommend going on placement to other students. |
 |
|||
Visit to BayerDr. Joaquin Urbina, Post-doctoral Research Associate, visited the Bayer Early Development laboratory site in Berlin and the Pharmaceutical Development site in Wuppertal, Germany, in March 2016. He visited the laboratory facilities there, and became familiar with the methods used to prepare amorphous solid solutions. The main highlight of his visit was a presentation of his project plan that he delivered in Wuppertal entitled “Predictive Assessment of Stability of Amorphous API Solid Solutions”. |
 |
|||
GSK SecondmentLed by CMAC research associate Dr Huaiyu Yang, CMAC has led and successfully completed an 11 hour continuous crystallisation of a live GSK product currently in clinical trials meeting the required product specifications. Following the CMAC workflow, Huaiyu gained the fundamental process understanding to develop the continuous process from the existing batch process. This resulted in separating nucleation (in a Y-mixer) from the growth units; in this case an MSMPR cascade. For this proprietary project. Huaiyu spent 6 months at GSK, Stevenage collaborating with Dr Flavien Susanne and Dr Mei Lee working in parallel to the ongoing batch development work. Dr Keddon Powell and Dr Cameron Brown of CMAC provided invaluable support to the campaign. Huaiyu will continue this work at Strathclyde but this time using an OBR for the residence time unit. |
 |
|||
GSK VisitDr Nicola Sewell, GSK, hosted CMAC research associate Nazer Rajoub for a week visit in April 2016 to carry out some characterisation of the previously produced agglomerates of a specific GSK model compound. The visit was part of the knowledge exchange and collaboration between CMAC and GSK. Experiments took place at the R&D GSK site in Stevenage and the manufacturing site in Ware. |
 |
|||
Visiting ResearchersIn late 2015 visiting PhD student Joost van den Ende from the Radboud University Nijmegen, finished his Short Term Scientific Mission (STSM) within COST Action CM1402 ‘Crystallise’. During his time at CMAC he simulated the attachment behaviour of organic molecules by probing the energy barriers upon leaving/approaching different crystal faces of DL- norleucine. These simulations have been done both in the presence and absence of water as a solvating medium. More knowledge about these energy barriers and their dependence on specific planes should lead to a better understanding of crystal growth on the molecular scale. Joost has been using Molecular Dynamics (MD) simulations and biased adaptations of of this technique. Joost is continuing his work on this topic in Nijmegen with the guidance of his supervisors Prof. Joop ter Horst and Dr. Miguel Jorge.
Weiwei Lee began his four month visit to CMAC in November 2015. Weiwei is currently a PhD student in the Intensified Reaction and Separation group in TUD, supervised by Prof. Joop H. ter Horst. His research topics are 1) Deracemization and 2) Electric Field Effect on Crystallization Behaviour. During his visit to CMAC, Weiwei studied whether it is possible to perform deracemization in a continuous mode and to investigate the agglomeration kernel in a racemic conglomerate slurry.
Ebenezer Ojo joined CMAC in March 2016 for three months, to continue the work he started at UCL on the iCON project. He has a degree in Chemical Engineering and MSc and MSc-Ph.D. degree in Industrial and Production Engineering and Biochemical Engineering respectively. He studied the impact of ultra scale- down technologies on crystal processing and recovery through a dead-end filtration process. He worked with Prof Alastair Florence, Dr Andrea Johnston, Dr Chris Price, and Sara Ottoboni. |
Figure: One particular molecular simulation in which a norleucine molecule (coloured in red) is pulled away from the [010]-plane of the alpha polymorph of DL-norleucine through a steered MD simulation. |
|||
Talent Pipeline 2016 |
||||
New Starts¦ Dr Stewart McCandless (National Facility Manager), from GSK ¦ Dr Keddon Powell (Research Associate, Strathclyde) from CMAC DTC Loughborough University ¦ Dr Joaquin Urbina, (Research Associate, Strathclyde) from Purdue, US ¦ Dr Claire MacDonald, (Business Development Manager, Strathclyde), from Vascutek, UK ¦ Kelly Etherson (Research Associate, Strathclyde) continuing from PhD in SIPBS at University of Strathclyde ¦ Javier Cardona (Research Associate, Strathclyde), continuing from PhD in Chemical and Process Engineering at Strathclyde ¦ Dr Akos Borsos (Research Associate, Loughborough), continuing from PhD at Loughborough University ¦ Rebekah Russell (Administrative Assistant, Strathclyde) continuing from University of Strathclyde ¦ Laura Harvey (Chemical Analysis Technician, Strathclyde) from Bioreliance ¦ Tanushree Mehta (National Facility Administrator, Strathclyde) from North Lanarkshire Council, UK ¦ Dr Alan Martin (X-ray Facility Research Technician, Strathclyde) from Scaled Solutions Ltd, UK ¦ Dr Deborah Bowering (Physical Analysis Research Technician) from Johnson Matthey, UK |
Next Steps¦ Tim Faehnrich, Freelance Accountant ¦ Dr Roy McBurney, Macquarie University, Australia ¦ Scott McPhee, City University of New York, US ¦ Jacqueline Brown, University of Strathclyde, UK ¦ Rachel Sheridan, science and engineering sector, UK ¦ Hannah McLachlan, Solid Form Solutions, UK ¦ Kate Wittering, Johnson Matthey, UK ¦ Dr Qinglin Su, Purdue, US ¦ Dr Nadeem Javid, University of Bradford, UK |
|||
|
|
||||
CMAC Publications from October 2015 to PresentAgnew, Lauren R., Cruickshank, Dyanne L., McGlone, Thomas, & Wilson, Chick C. (2016). Controlled production of the elusive metastable form II of acetaminophen (paracetamol): a fully scalable templating approach in a cooling environment. Chemical Communications. doi: 10.1039/C6CC01032F Bhardwaj, Rajni M, Yang, Huaiyu, & Florence, Alastair J. (2016). Crystal structure of the co-crystal butylparaben–isonicotinamide (1/1). Acta Crystallographica Section E: Crystallographic Communications, 72 (1), 53-55. doi: 07/S2056989015023518 Briggs, Naomi E. B., Schacht, Ulrich, Raval, Vishal, McGlone, Thomas, Sefcik, Jan, & Florence, Alastair J. (2015). Seeded Crystallization of β-l-Glutamic Acid in a Continuous Oscillatory Baffled Crystallizer. Organic Process Research & Development, 19(12), 1903-1911. doi: 10.1021/acs.oprd.5b00206 Evora, Antonio O. L., Castro, Ricardo A. E., Maria, Teresa M. R., Silva, M. Ramos, ter Horst, J. H., Canotilho, Joao, & Eusebio, M. Ermelinda S. (2016). Co-crystals of diflunisal and isomeric pyridinecarboxamides - a thermodynamics and crystal engineering contribution. CrystEngComm. doi: 10.1039/C6CE00380J Grothe, E., Meekes, H., Vlieg, E., ter Horst, J. H., & de Gelder, R. (2016). Solvates, Salts, and Cocrystals: A Proposal for a Feasible Classification System. Crystal Growth & Design. doi: 10.1021/acs.cgd.6b00200 Klapwijk, Anneke R., Simone, Elena, Nagy, Zoltan K., & Wilson, Chick C. (2016). Tuning crystal morphology of succinic acid using a polymer additive. Crystal Growth & Design. doi: 10.1021/acs.cgd.6b00465 Marshall, R. J., Griffin, S. L., Wilson, C., & Forgan, R. S. (2016). Stereoselective Halogenation of Integral Unsaturated C-C Bonds in Chemically and Mechanically Robust Zr and Hf MOFs. Chemistry, 22(14), 4870-4877. doi: 10.1002/chem.201505185 Martinez-Marcos, Laura, Lamprou, Dimitrios A., McBurney, Roy T., & Halbert, Gavin W. (2016). A novel hot-melt extrusion formulation of albendazole for increasing dissolution properties. International Journal of Pharmaceutics, 499(1–2), 175-185. doi: http://dx.doi.org/10.1016/j.ijpharm.2016.01.006 McLachlan, Hannah, & Ni, Xiong-Wei. (2016). On the effect of added impurity on crystal purity of urea in an oscillatory baffled crystallizer and a stirred tank crystallizer. Journal of Crystal Growth, 442, 81-88. doi: http://dx.doi.org/10.1016/j.jcrysgro.2016.03.001 Ni, Xiong-Wei, & McLachlan, Hannah. (2016). An Investigation into Parameters Affecting Crystal Purity of Urea in a Stirred Tank and an Oscillatory Baffled Crystallizer. Chemical Engineering Communications, null-null. doi: 10.1080/00986445.2016.1154851 Powell, K. A., Croker, Rielly, C. D., & Nagy, Z. K. (2016). PAT-based design of agrochemical co-crystallization processes: A case-study for the selective crystallization of 1:1 and 3:2 co-crystals of p- Toluenesulfonamide/triphenylphosphine oxide. Chemical Engineering Science. doi: http://dx.doi.org/10.1016/j.ces.2016.06.005 Powell, K. A., Saleemi, A. N., Rielly, C. D., & Nagy, Z. K. (2015). Periodic steady-state flow crystallization of a pharmaceutical drug using MSMPR operation. Chemical Engineering and Processing: Process Intensification(0). doi: http://dx.doi.org/10.1016/j.cep.2015.01.002 Powell, Keddon A., Saleemi, Ali N., Rielly, Chris D., & Nagy, Zoltan K. (2016). Monitoring Continuous Crystallization of Paracetamol in the Presence of an Additive Using an Integrated PAT Array and Multivariate Methods. Organic Process Research & Development. doi: 10.1021/acs.oprd.5b00373 Ross, S. A., Lamprou, D. A., & Douroumis, D. (2016). Engineering and manufacturing of pharmaceutical co-crystals: a review of solvent-free manufacturing technologies. Chemical Communications. doi: 10.1039/C6CC01289B Sayin, R., Martinez-Marcos, L., Osorio, J. G., Cruise, P., Jones, I., Halbert, G. W., . . . Litster, J. D. (2015). Investigation of an 11mm diameter twin screw granulator: Screw element performance and in-line monitoring via image analysis. Int J Pharm. doi: 10.1016/j.ijpharm.2015.09.024 Siddique, Humera, Brown, Cameron J., Houson, Ian, & Florence, Alastair J. (2015). Establishment of a Continuous Sonocrystallization Process for Lactose in an Oscillatory Baffled Crystallizer. Organic Process Research & Development, 19(12), 1871-1881. doi: 10.1021/acs.oprd.5b00127 Srai, Jagjit Singh, Harrington, Tomás, Alinaghian, Leila, & Phillips, Mark. (2015). Evaluating the potential for the continuous processing of pharmaceutical products—a supply network perspective. Chemical Engineering and Processing: Process Intensification, 97, 248-258. doi: http://dx.doi.org/10.1016/j.cep.2015.07.018 Srirambhatla, Vijay K., Guo, Rui, Price, Sarah L., & Florence, Alastair J. (2016). Isomorphous template induced crystallisation: a robust method for the targeted crystallisation of computationally predicted metastable polymorphs. Chemical Communications. doi: 10.1039/C6CC01710J Xiouras, Christos, Van Aeken, Jasper, Panis, Joris, Ter Horst, Joop H., Van Gerven, Tom, & Stefanidis, Georgios D. (2015). Attrition-Enhanced Deracemization of NaClO3: Comparison between Ultrasonic and Abrasive Grinding. Crystal Growth & Design, 15(11), 5476-5484. doi: 10.1021/acs.cgd.5b01108 Yang, Huaiyu, Yu, Xi, Raval, Vishal, Makkawi, Yassir, & Florence, Alastair. (2016). Effect of Oscillatory Flow on Nucleation Kinetics of Butyl Paraben. Crystal Growth & Design, 16(2), 875-886. doi: 10.1021/acs.cgd.5b01437 Chris Price has authored 2 book chapters: “Application of Ultrasound in Crystallisation (Sonocrystallisation) ” and “Continuous Pharmaceutical Crystallisation from Solution”. They will be published in “Engineering Crystallography: From Molecule to Crystal to Functional Form” the proceedings of the 48th NATO crystallographic course at Ettore Majorana Centre, Erice, Italy 2015 |
||||
|
|
||||
PresentationsAdelakun, J., (2016, June) Kinetics Study of Melt Crystallisation (Palm Oil as Case study) at the joint BACG2016 & CGOM12 conference, Leeds, UK Agnew, L., McGlone, T., Wilson, C.C., (2016, June) Towards Multi-component Templated Continuous Crystallisation of Paracetamol Form II at the joint BACG2016 & CGOM12 conference, Leeds, UK Agnew, L., McGlone, T., Wilson, C.C., (2016, April) Towards Multi-component Templated Continuous Crystallisation of Paracetamol Form II at BCA Spring Meeting, Nottingham, UK Aleksiev, G., (2016, May) Sustainability trends in Pharmaceutical Supply Chains at POMS 2016, Orlando, Florida Connor, L., (2016, May) Crystal Engineering of Multicomponent Constructs and the Impact of High Pressure at Erice 2016 Ejim., L, (2016, June) A Design of Experiment (DoE) Approach to Optimize the Inner Geometry of Baffled Meso-scale Tubes for Continuous Crystallization at PIN, Newcastle Fysikopoulos, D., (2016, June) Model Reliability of multi-dimensional Population Balance Models for Crystallization at PSE@ResearchDayUK Symposium Harrington, T., (2016, May) Pharmaceutical Industry Review I: Context, Trends and Drivers at POMS 2016, Orlando, Florida Kendall, T., (2016, June) Filtration Suppresses Laser Induced Nucleation of Glycine in Aqueous Solutions at the joint BACG2016 & CGOM12 conference, Leeds, UK Mendez, C., (2016, April) Quantification of the Homogeneity of Particle Size Distributions in Continuous Wet Granulation at APS International PharmSci 2016 Ottoboni, S., Chrubasik,M., Bruce,L.M., Nguyen, H., Johnston, B.,Florence, A., Price, C., (2016, June) The impact of paracetamol impurities on face-specific properties: investigating the surface of single crystals using TOF-SIMS at the joint BACG2016 & CGOM12 conference, Leeds, UK Paladino, E., (2016, April) Application of Time of Flight - Secondary Ion Mass Spectrometry (ToF-SIMS) in the Analysis of Pharmaceutical Solid Forms at APS International PharmSci 2016 Todorova-Aleksiev, L., (2016, May) Design and Analysis of a Pharmaceutical Continuous Flow Manufacturing System Using Value Stream Mapping at POMS 2016, Orlando, Florida |
||||
|
|
||||
|
Posters Cousen, A., (2016, June) Selective Preparation of Polymorphic, Stoichiometric and Enantiomeric Multi-component Materials. Poster presented at the joint BACG2016 & CGOM12 conference, Leeds, UK. Ejim, L., (2016, June) A Design of Experiment (DoE) Approach to Optimize the Inner Geometry of Baffled Meso-scale Tubes for Continuous Crystallization. Poster presented at the joint BACG2016 & CGOM12 conference, Leeds, UK. Fysikopoulos, D., (2016, June) Parameter Identification, Reliability and Model Discrimination of Population Balance Models for Crystallization. Poster presented at the joint BACG2016 & CGOM12 conference, Leeds, UK. Kimuli, E., (2016, June) Determining Residence time distribution and axial dispersion coefficient in meso-scale oscillatory baffled crystallizer using a Computational fluid dynamics approach. Poster presented at the joint BACG2016 & CGOM12 conference, Leeds, UK. McGinty, J., Price, C., & Sefcik, J. (2016, June). Continuous Reactive Crystallisation for Control of Salt Formation Processes. Poster presented at the joint BACG2016 & CGOM12 conference, Leeds, UK. Paladino, E., (2016, June). Application of Time of Flight - Secondary Ion Mass Spectrometry (ToF-SIMS) in the analysis of drug-loaded electrospun nanofibers. Poster presented at UKSAF Summer meeting, University College Dublin Svoboda, V., (2016, June) Continuous Antisolvent Co-crystallization of Benzoic Acid and Isonicotinamide. Poster presented at the joint BACG2016 & CGOM12 conference, Leeds, UK Steendam, R.R.E., Bruiglia, M., Sefcik, J., ter Horst, J.H., (2016, June). Primary and Secondary Nucleation of Chiral Crystals. Poster presented at the joint BACG2016 & CGOM12 conference, Leeds, UK. Yang, H.; Yu, X. ; Raval, V. ; Makkawi, Y.; Florence, A.; (2016, June) Poster presentation, Shear rate and nucleation rate in oscillatory flow crystallizer Poster presented at the joint BACG2016 & CGOM12 conference, Leeds, UK. |
||||
|
|
||||
Come and See Us at These Events05-07 September 2016 7th APS International PharmSci Conference (website here) 26-27 September 2016 2nd Annual Continuous Symposium CMAC-MIT (website here) 30 Nov - 01 Dec 2016 I2APM International Symposium & Satellite Training Day For a full list of external events of interest to CMAC please click here |
||||
Follow us www.cmac.ac.uk
|
||||
| |
||||
|
If you have any comments or feedback, please send onto the CMAC team Unsubscribe to this newsletter please click here |
||||







