This research explores possibilities to align future value network configurations and disruptive shifts in manufacturing and information technologies (e.g. digitalisation, continuous manufacturing) to enable novel routes to medicines production, and deliver added value to ‘end-users’, i.e. payers and patients. While evidence exists that continuous processing delivers financial benefits for single-purpose plants, a business case for transformation assessing the resultant impact across the end-to-end (E2E) value chain is needed for such a technology to be better understood and quantified, and for the upstream and downstream linkages to emerging continuous process technologies (e.g. in synthesis and work-up, filtration, drying, secondary processing) to be effectively exploited.
To assess value network reconfiguration opportunities enabled by targeted technology interventions, a 4-step approach developed as part of this research at Cambridge, is applied to explore opportunities with respect to new and established markets where interventions in continuous crystallisation and adjacent unit operations may create attractive value network opportunities.
Pre-screening
In the case of more established generic products, we often choose to examine traditional batch processing routes that may be considered uneconomical in delivering on future requirements and changing markets. As part of CMAC phase II activities, paracetamol was selected as a case demonstrator.
Current state mapping and future state models
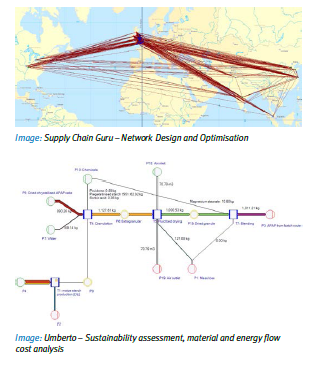
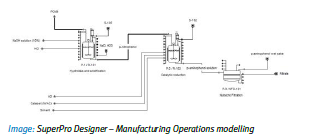
The proposed approach extends prior work on industrial systems analysis and supply chain mapping techniques, and provides a characterisation and visualisation of the ‘current state’ for the candidate drug product, in terms of unit operations and the supply network. This mapping exercise scopes out those unit operations where an existing batch production process may be amenable to a series of continuous technologies, in terms of current state and future potential. It is estimated that up to 80% of production, currently located in India, may remain via a p-nitrochlorobenzene (PNCB) batch route. Opportunities were identified in the areas of inventory, flexibility (both mix and volume), process control, sustainability, and quality (purity), and future state scenarios for processes technologies and network configurations were derived accordingly.
An E2E assessment of benefits and emerging patterns is carried out in a variety of product/delivery/patient scenarios. Key to this our capability to model complex systems, using Cambridge’s Network Design Process Laboratory suite of tools.
Building the business case for transformation Finally, we build the business case for transformation. This involves integrating (1) outputs from the previous steps, (2) business context and viability considerations - balancing the transformation versus the investments required (potential impact on revenue, margin, inventory reduction), informed by key economic evaluation criteria in terms of batch versus continuous (direct fixed capital, plant throughput, manufacturing cost, unit production cost), and (3) technology readiness inputs (from supporting technology roadmaps and specific technology interventions with realistic timescales). Growing demand may contribute to an attractive batch-to-continuous transformation agenda in certain scenarios (e.g. a move to more localised and market/country-specific supply) where continuous crystallisation may support smaller plant footprints, with associated capital cost reductions.