

CMAC’s vision to transform medicines manufacturing requires development of innovative, modular, integrated continuous manufacturing processes for drug substance and drug product. The end-to-end modular lab scale microfactories are being developed within the Hub to provide a rapid prototyping capability to control, measure and optimise critical transformations across multiple length-scales spanning crystal and particle engineering, structured product and dosage form generation; managing variable material properties and increased product complexity. Input from the Predictive Design & Digital Twin, are used to design, build and operate flexible, integrated continuous manufacturing process chains at scale (kgs/day). A key deliverable is to enable simplified process chains targeting the processes and transformations that improve manufacturability of the drug into a dosage form suitable for performance in the patient. By developing integrated continuous direct to dose approaches we avoid multiple unit operations and scale up steps.
The Hub has the following microfactory research projects ongoing:
Microfactory 1 (MF1) looks at product process archetypes that go from crystallisation through isolation and drying and then take dry powder into a hot melt extruder to combine the drug with polymer and then process the extruded drug polymer mixture via either 3D printing or injection moulding to produce a solid dosage form. The polymer is selected based on properties of the API and desired performance in vivo.
Microfactory 3 (MF3) addresses the challengesprovided by drug compounds with difficult morphologies. An example of how this can be addressed is processing API with needle-like crystals by using a spherical agglomeration process to change the particle aspect ratio prior to isolation and drying, then using the dried powder for producing tablets by direct compression.

|
Product Process Archetypes CMAC has identified scenarios that combine addressing challenging physical properties with specific continuous manufacturing chain processes. We have termed these scenarios product process archetypes (PPAs). We will target PPAs where integrated continuous processing will deliver benefits. Underpinning this is the idea that there are types of particle that will likely have an ideal type of continuous manufacturing process that delivers desired product performance. For example in MF3 the PPA3 has “needle-like” crystals that usually need to be processed in some way to give particles that are able to be handled easily and then formulated into products with desired performance. |
PPA1 will deliver a microfactory that combines crystallisation, isolation, extrusion and 3D-printing or injection moulding of a drug/polymer suspension, and fits with MF1.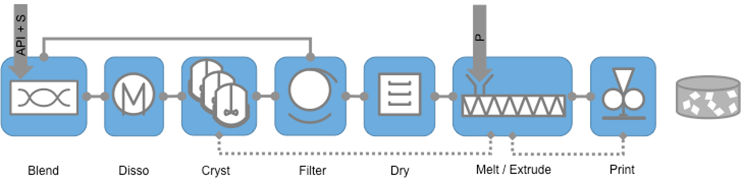
PPA 2 is very similar to PPA1 but addresses API with poor molecular bioavailability as per figure below. It aslo fits with MF1.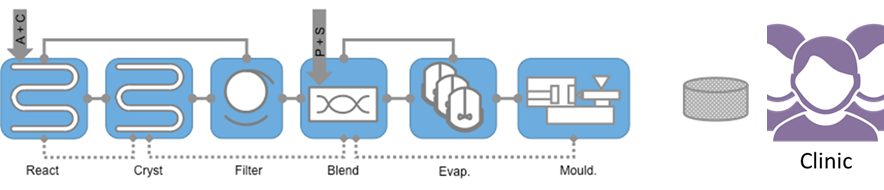
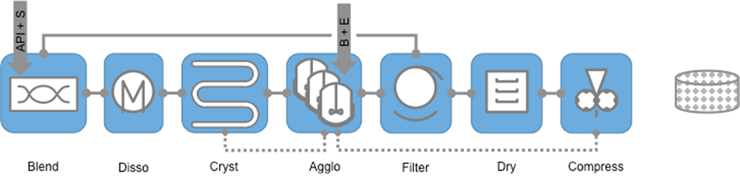
PPA3 that will deliver MF2 by combining continuous crystallisation, spherical agglomeration, and direct compression to produce the final dosage form and will address difficult morphology issues (e.g. needle like crystals).